Development and Validation of a Novel and Simple Isocratic HPLC Method for Simultaneous Estimation of Nelfinavir and Quercetin in Patented Pharmaceutical Formulation
HPLC Method for Nelfinavir and Quercetin Estimation
DOI:
https://doi.org/10.61920/jddb.v1i01.25Keywords:
RP-HPLC, Validation, Nelfinavir, QuercetinAbstract
Objective: A novel, selective, precise, and accurate reverse phase High Performance Liquid Chromatographic method for estimation of Nelfinavir(NEL) and (Quercetin) was developed and validated according to ICH guidelines
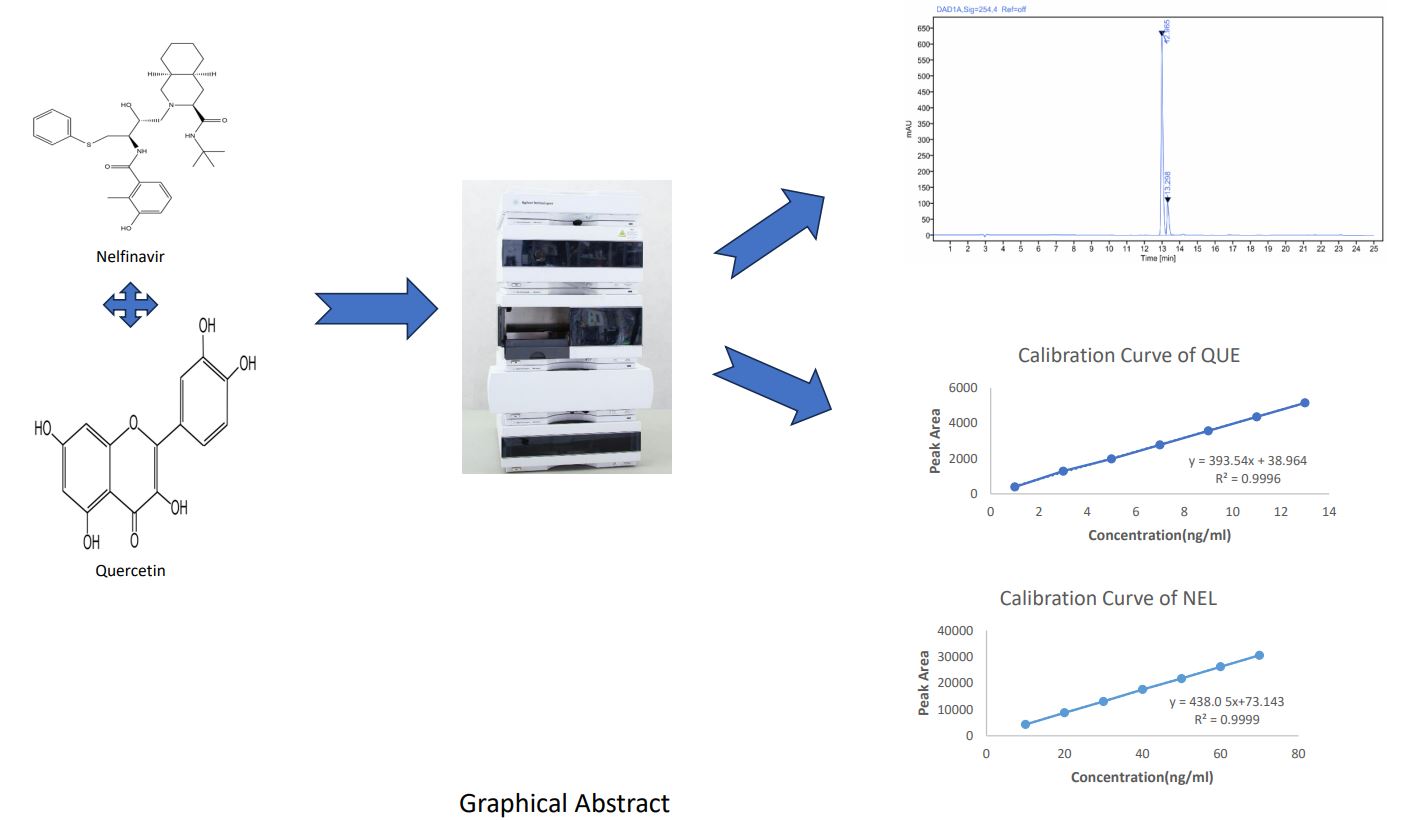
Method: HPLC method was developed using C18, (150mm × 4.6mm, 3.5μm) column with 1% Acetic acid in water: Methanol (45:55 v/v)as a mobile phase at a flow rate of 0.8mL/min and eluents were detected at 284 nm.
Results:
The calibration curves were linear over the concentration range of 10 to 70 ng/mL (R2 =0.9999) for Nelfinavir and 1 to 13 ng/mL (R2 = 0.9996) for Quercetin. The average retention time of Nelfinavir and Quercetin was found to be 12.96 min and 13.29 min respectively. Average percentage recoveries of Nelfinavir and Quercetin were 99.99 ± 0.34 and 100.52± 0.28 %, respectively. LOD and LOQ of proposed method were found to be 0.1195 ng/ml and 0.3623 ng/ml for Nelfinavir respectively whereas for Quercetin, said values were 0.0372 ng/ml and 0.1129 ng/ml respectively. Intra- and inter-day precision values (% RSD) of proposed method were less than 2%.
Conclusion: A simple, precise, accurate, linear and rapid RP-HPLC method was developed for simultaneous estimation of Nelfinavir and Quercetin and validated as per ICH guidelines. The results suggest that the developed method can be applicable in routine estimation of Nelfinavir and Quercetin in bulk as well as pharmaceutical formulation.