Analytical Method Development and Validation for Venlafaxine Hydrochloride: UV-Visible Spectrophotometric Estimation in Bulk and Pharmaceutical Dosage Forms
Analytical Method development for Venlafaxine Hydrochloride
Keywords:
Antidepressant, Venlafaxine HCL, Analytical Validation, UV visible spectrophotometer, Method developmentAbstract
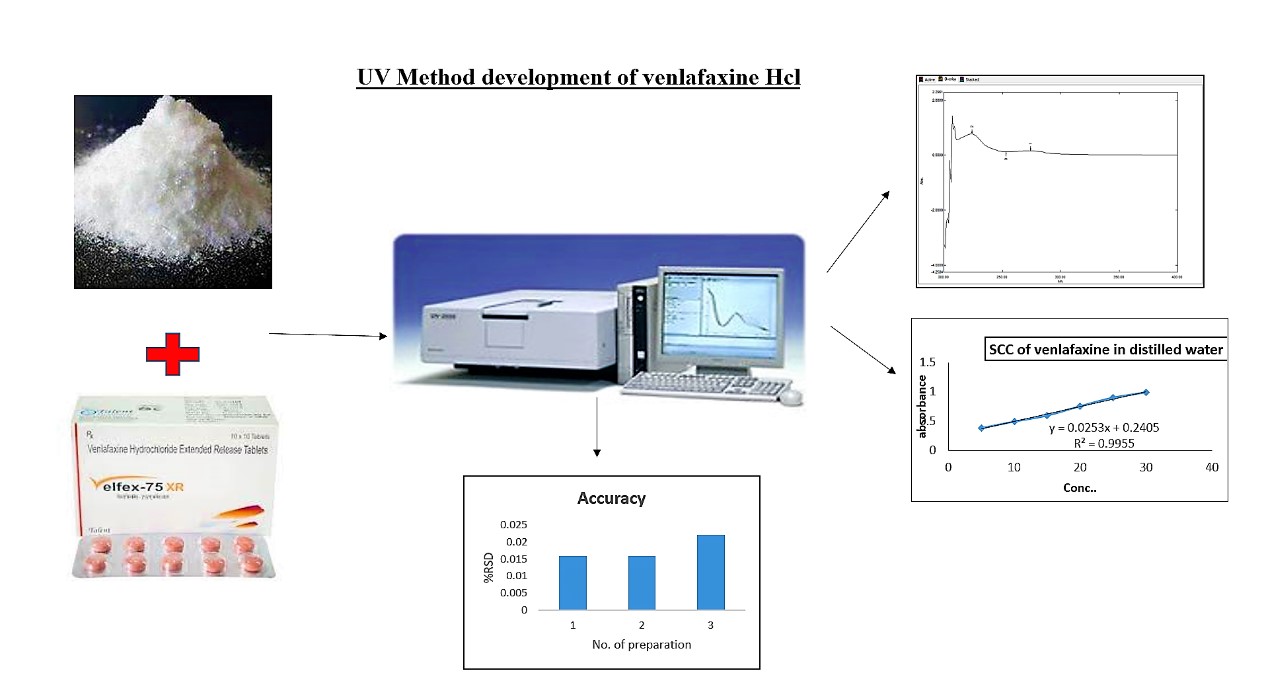
This abstract outline the development and validation of a UV-visible spectrophotometric method for the precise estimation of Venlafaxine HCl, a widely used antidepressant, in both bulk and pharmaceutical formulations. Employing purified water as the solvent and identifying the lambda max at 224.60 nm, the method demonstrated excellent linearity over the concentration range of 5-35 μg/ml. The linear regression analysis yielded a remarkable correlation coefficient (R2 = 0.999) and robust mean recovery rates ranging from 99.00% to 100.0%.
This cost-effective method was effectively applied to assess the quality of Venlafaxine HCl in both active pharmaceutical ingredients and commercially available tablets. The validation process, adhering to ICH guidelines and covering Linearity, Repeatability, Precision, Accuracy, Ruggedness, and LOD & LOQ, underscored the statistical reliability of the method. Recovery studies further confirmed the method's dependability for routine analysis of Venlafaxine HCl in bulk and marketable formulations. In conclusion, this study contributes significantly to the repertoire of analytical methods available for Venlafaxine HCl assessment, providing a swift, reliable, and cost-effective alternative suitable for routine quality control applications.