Hepatotoxicity Induced by Anti-Tuberculosis Medications: Exploring Causes, Risk Factors, and Potential Mitigation Strategies
Hepatotoxicity in Anti-Tuberculosis Therapy: Causes, Risks, and Mitigation
DOI:
https://doi.org/10.61920/jddb.v1i01.16Keywords:
Drug-induced liver injury, Hepatotoxicity, Genetics, Tuberculosis, PathogenesisAbstract
Tuberculosis (TB), a global health concern, remains a leading cause of mortality, with 9 million new cases and 1.7 million deaths reported in 2004. This review delves into a critical facet of TB treatment-antituberculosis drug-induced hepatotoxicity (ATDH). Beyond the direct threat to patients' health, ATDH hampers the efficacy of the prescribed medications. Recognized as a prime contributor to idiosyncratic hepatotoxicity worldwide, anti-TB drugs present a complex interplay of treatment regimens, hepatotoxicity thresholds, patient cohorts, and monitoring protocols. The study comprehensively addresses the incidence, pathophysiology, and clinical dimensions of ATDH, emphasizing the metabolism and toxicity mechanisms of key drugs-isoniazid, rifampicin, and pyrazinamide. Occurring in 2% to 28% of individuals, hepatotoxicity induced by first-line anti-TB drugs can manifest as immediate liver damage, failure, or even necessitate emergency transplantation. Notably, ethambutol, isoniazid, pyrazinamide, rifabutin, rifampin, and rifapentine fall under this category. In conclusion, ATDH spans a spectrum from asymptomatic enzyme elevations to life-threatening liver failure. Despite widespread usage, the etiology of ATDH remains elusive. The review underscores the importance of investigating drug-related, host genetic, and environmental factors contributing to hepatotoxicity. Emphasizing the need for future research, the article calls for a deeper understanding of the mechanistic underpinnings, exploration of genetic risk factors, and the development of shorter, safer TB treatment regimens to mitigate this significant challenge in global health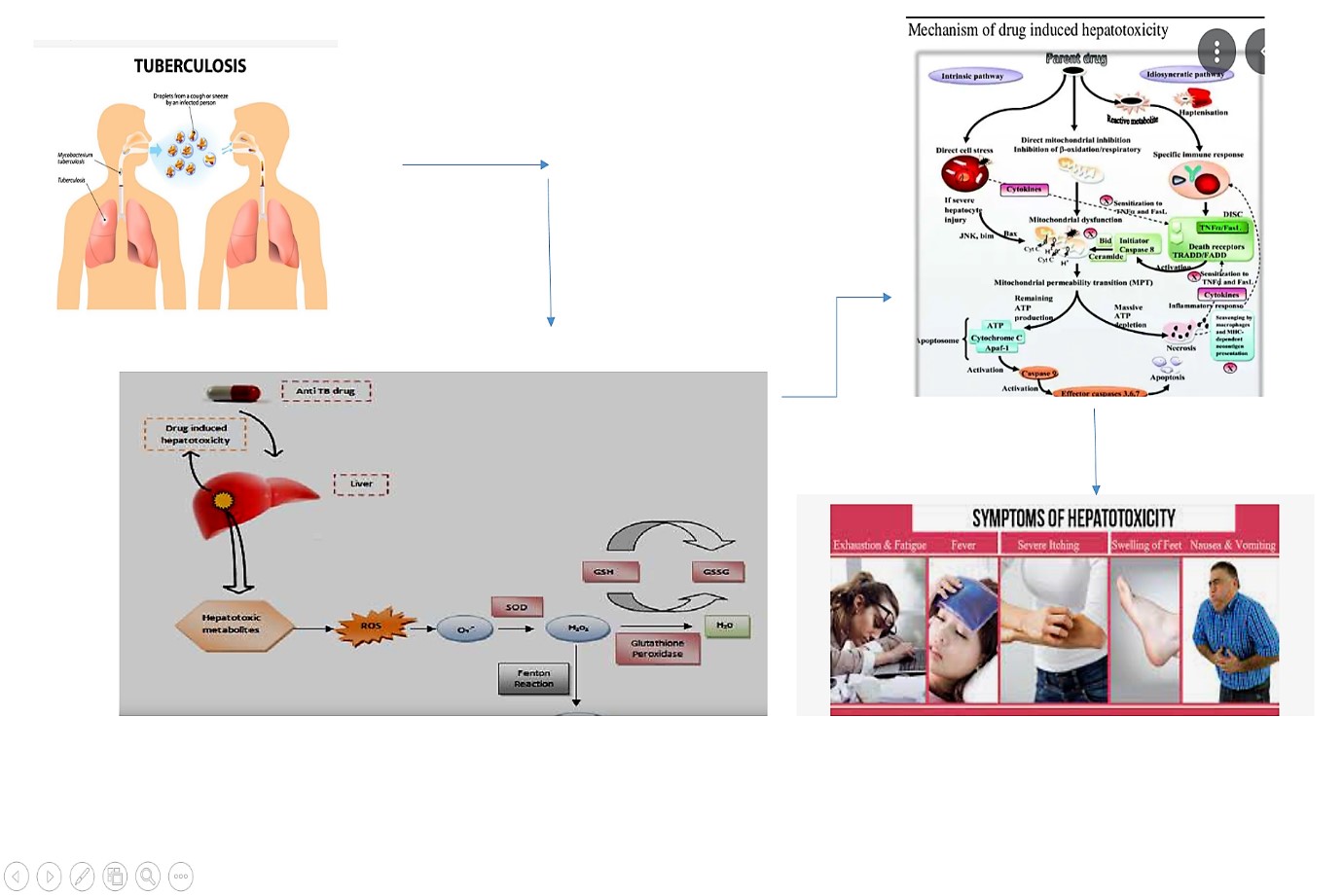
Downloads
Additional Files
Published
2024-04-25
How to Cite
Zade, D., & Raut, P. (2024). Hepatotoxicity Induced by Anti-Tuberculosis Medications: Exploring Causes, Risk Factors, and Potential Mitigation Strategies: Hepatotoxicity in Anti-Tuberculosis Therapy: Causes, Risks, and Mitigation. Journal of Drug Delivery and Biotherapeutics, 1(01), 1–14. https://doi.org/10.61920/jddb.v1i01.16
Issue
Section
Articles



